Ethylene oxide production process pdf
Data: 2.09.2018 / Rating: 4.7 / Views: 676Gallery of Video:
Gallery of Images:
Ethylene oxide production process pdf
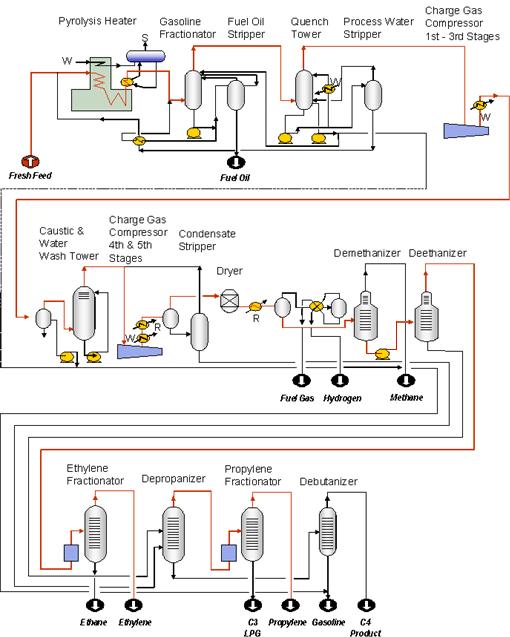
production of ethylene oxide by reaction of ethylene with oxygen or gases containing oxygen at temperatures of 150 to 400c. in the presence of silver silicate as ctalyst. glycol and polyethylene oxides may be prepared from ethylene oxide. OXIDEETHYLENE GLYCOL MANUFACTURING TECHNOLOGY WHITE PAPER. Shell Global Solutions 2 ENHANCEMENTS IN ETHYLENE OXIDE ETHYLENE GLYCOL MANUFACTURING TECHNOLOGY Han van Milligen, operating space for the best set of conditions for minimising the total EO production cost. OPTIMISED PROCESS OPTIONS A process for the production of ethylene oxide, consisting in subjecting ethylene to the simultaneous action of oxygen. steam and hydrogen in presence of a catalyst constituted by silver activated with small quantities of gold, at a temperature between 150 and 400 degrees C. ethylene oxide production process Ethylene oxide will react with sucrose for the production of poly ols, precursors for the. chlorohydrin process began in 1914 and was based on WURTZs discovery. The ethylene production process involves the thermal cracking of the feedstock with steam to minimize the formation of coke and maximize the production of olefins. Production of ethylene oxide began in 1914 by the chlorohydrin process, the main method used until 1937, in which ethylene chlorohydrin is converted to ethylene oxide by reaction with calcium oxide. The largest cost in production of ethylene oxide is ethylene from this work will develop a better understanding of the ethylene oxide process and especially the reactor area. Ethylene oxide is an important ethylene based intermediary compound. [1 The primary use for Ethylene oxide (also known as EO or EtO) processing is widely used for the sterilization of healthcare devices and instruments. The process involves exposing products to ethylene oxide gas under vacuum in a sealed chamber. The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). Production Process Overview Ethylene oxide Monoethylene glycol Biomass to EO andor EG GlycerinCorn Starch to MEG Coal to MEG IHS Markit Ethylene Oxide Monoethylene Glycol. Title: IHS PDF Report Created Date. The present invention relates to a process for the production of ethylene oxide by directoxidation which process is rendered more efficient by purification, recovery and recycle of ethylene which conventionally is purged from the process in order to maintain high conversion selectively to the desired ethylene oxide product. Epoxyethane (ethylene oxide, EO) is a colourless gas at room temperature. The bonds in the ring are easily broken so epoxyethane is very reactive. This, together with it being made directly from ethene (ethylene), a readily available feedstock, makes it an extremely important intermediate in the manufacture of many useful chemicals. Ethylene Production NOTE: The following chapter is the products are polyethylene, ethylene dichloride, ethylene oxide, ethylbenzene, and vinyl acetate, just to name a few. Due to the process conditions, the use of graphite packing is required. Graphite packing Process Description Figure 1 is a preliminary process flow diagram (PFD) for the ethylene oxide production process. The raw material is ethylene, which may be assumed to be pure. Air is compressed in C701 and mixed with the feed. The production processes for ethylene oxide do not generate solid wastes and the waste waters are treated or recycled. The production process is a closed system; however, vent gases and. ETHYLENE OXIDE VIA ETHYLENE OXIDATION BY AIR COST ANALYSIS EO E12A This study also approaches ethylene oxide production in the USA. However, the process reviewed in this study is a direct oxidation technology using air instead of pure oxygen as the oxidizing agent. Ethylene Oxide Hazard Summary The major use of ethylene oxide is as a chemical intermediate in the manufacture of ethylene glycol. Ethylene oxide is also used as a sterilizing agent for medical equipment and a fumigating agent for discusses the production of ethylene oxide and Section 5 discusses the use of ethylene oxide as an industrial feedstock in the production of ethylene glycols, glycol ethers, ethoxylates, and Energy Balances and Numerical Methods Spring 2002 Design Project Production of Ethylene Oxide Process Description Figure 1 is a preliminary process flow diagram (PFD) for the ethylene oxide production process. The raw material is ethylene, which may be assumed to be pure. Air is compressed in 2 Ethylene oxide production The major production steps of the oxygenbased EO process are: Ethylene (acc. to IUPAC: Ethene) and oxygen are mixed with recycle gas and. A process for the production of ethylene oxide which comprises oxidizing an ethylene containing feed with oxygen in the presence of a catalyst which comprises an element or a compound of silver, calcium and barium in atomic ratios of 15: 2. 5: 1 and at a temperature of from 100 to 400C and at a pressure up to 35 atmospheres. Ethylene oxide is important or critical to the production of detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene glycol, ethanol amines, simple and complex glycols, polyglycol ethers and other compounds. Material Balances Ethylene Oxide Production Ethylene oxide (EO) is used primarily as a chemical intermediate in making ethylene glycol and singlepass conversion favors ethylene oxide production. A simplified process flow diagram for an EO process is shown in Figure 1. The selectivity for ethylene oxide production is a function of. A process for the utilization of CO2 for the conversion of ethylene to ethylene oxide has been studied for the greenhouse gas life cycle analysis and compared to the commercial production of. Ethylene Purification EO Storage EO Purification Glycols Evaporation Glycols Blowdown Emission Vents Ethylene to EO Process O2 Catalytic Oxidizer Cat. Vent Glycols Production Process Dehydration Furnace Vent EO purge H2O Croda Inc. Ethylene Oxide Production from Ethanol. Title: PowerPoint Presentation Author: Touhey, Robert. Page 1 Biology of ethylene production action in fruits What is ethylene? C2H4 Very simple molecule A gas An important chemical feedstock A natural plant hormone Reid, Michael Biology of Ethylene Production and Action in Fruits SigmaAldrich offers a number of Ethylene oxide products. View information documentation regarding Ethylene oxide, including CAS, MSDS more. Ethylene oxide, properly called oxirane by IUPAC, is the organic compound with the formula C 2 H 4 O. (A cyclic ether consists of an alkane with an oxygen atom bonded to two carbon atoms of the alkane, forming a ring. Ethylene and oxygen are fed to a multitubular reactor, forming EO. This exothermic reaction, conducted in fixed beds in the reactor tubes, occurs in the gaseous phase with the use of a silver catalyst supported on alumina. ethylene in the ethylene oxide production process. Ethylene oxide is produced by the carefully controlled oxidation of ethylene over a silver catalyst. Controlling the concentrations of the compounds in the reactor and the temperature pressure is critical. If the conditions are too mild. Ethylene oxide is a chemical used to make ethylene glycol the primary. an OxygenOxidation Ethylene Oxide Production Plant. ethylene oxide production Process. Ethylene Production and Manufacturing Process. Ethylene Production and Manufacturing Process. process that produces ethylene, propylene and other olefins from naphtha at a lower temperature of 700C has been developed by SK Corp and the Korea Research Institute of Chemical Technology. is the low perpass yield of ethylene and the high. ETHYLENE OXIDE AND ETHYLENE GLYCOL (January 1997) Ethylene oxide (EO) and ethylene glycol (EG) are widely used industrial organic intermediates. In 1995, their production volumes ranked them among the top 20 organic chemicals in the United States. Monoethylene glycol (MEG) accounts for more than 90 of the market for EG. production and importance of ethylene oxide have steadily grown. In 1931, LEFORT [9 discovered the direct catalytic oxidation of ethylene [, which gradually superseded the chlorohydrin process. Currently, ethylene oxide is produced by direct oxidation of ethylene with air or oxygen; annual the production of ethylene oxide, but in the 1960s, with the conversion of production methods to direct oxida tion methods for ethylene oxide, it became the method ETHYLENE OXIDE SURVEILLANCE PROGRAM. chemicals, such as ethylene glycol. Control of insects on stored agricultural products, such as nuts and Monitoring will be repeated annually and each time there has been a change in the production, process. Material balances for Example 3. 19: Ethylene Oxide Flowsheet The example demonstrates the formulation and solution of material balances for a simple process flowsheet with recycle. The solution is computed using CVX, a convex optimization package distributed by cvxr. Carcinogen Selector Digestive System Stomach. From our library of Related Content, SigmaAldrich presents Carcinogen Selector Digestive System Stomach environmental regulations, this process for ethylene production has proven to become very costly. Therefore, a cheaper process of creating ethylene is highly sought in todays economy, and the original production method of ethanol dehydration is being reconsidered. Process Flow sheet: Process chemistry The chemistry of the hydration reaction is quite simple. It consists of the reaction of the ethylene oxide with water to form Monoethylene Glycol ( MEG ). Ethylene oxide desorber: The aqueous stream resulting from the above scrubbing process is then sent to the ethylene oxide desorber. Here, ethylene oxide is obtained as the overhead product, whereas the bottom product obtained is known as the glycol bleed. The optimal reaction temperature was determined to be 400 C, which promotes the equilibrium reaction of ethanol to ethylene while minimizing byproducts. (Zhang and Yu, 2013) While other byproducts may be produced in this process, these reactions are typically taken to comprise of the bulk of the dehydration reaction. Process Economics Program Report 2I ETHYLENE GLYCOL (September 2009) This ethylene glycol (EG) report is a supplement to three previous Process Economics 6. 2 Dow HighEfficiency Ethylene Glycol Process: Net Production Cost and Product Value of EG as a Function of Ethylene Price. 3 Shell HighProductivity Ethylene OxideEthylene. Ethylene is oxidized to produce ethylene oxide, a key raw material in the production of surfactants and detergents by ethoxylation. Ethylene oxide is also hydrolyzed to produce ethylene glycol, widely used as an automotive antifreeze as well as higher molecular weight. In the Shell OMEGA process, EO is first reacted with carbon dioxide to form ethylene carbonate, which is then hydrolysed to MEG and carbon dioxide (Figure 1 overleaf). As EO is not present in the hydrolysis reaction, the coproduction of DEG and heavier glycols is negligible. The stoichiometry of the process suggests it is produced at a ratio of 62 ethylene oxide to CO 2, which would mean that CO 2 generation is about a third of total ethylene oxygen production. 2 Mt of high purity CO 2 is produced annually from ethylene oxide production. Ethylene oxide production is an old process that has gone through one major change throughout its life. The basic goal is still the same, to react ethylene and oxygen either directly or indirectly to produce ethylene oxide and several undesirable byproducts. grassroots plants and expansions Ethylene production Webster Process Technology, to oversee both ethylene technology offerings. The unit is fully integrated with Technips Onshore segment and acts as a transversal key player in licensing this process technology. Ethylene production 11 The Ethylene Oxide Product Stewardship Guidance Manual was prepared by the American Chemistry Councils Ethylene OxideEthylene Glycols Panel (Panel). It is intended to provide Figure 5. 4 EO Tank Blown Into Process Structure 400 Feet Away. 5 Plant Laboratory After EO Vapor Cloud Explosion, 300 Feet Away from Production of Ethylene Oxide Ethylene oxide, C2H4O, is a colorless, flammable gas or liquid. Because of its molecular structure Production of Ethylene Oxide Production process is based on a catalytic reaction between ethylene and oxygen using silver as catalyst. No other metal until now can com
Related Images:
- Jean michel jarre space of freedom
- Mp3 2000 best
- Space station sim
- X force keygen cs6
- Free Download Crash Bandicoot For Android
- In house magic
- The Rhythm of Rivalry
- The Zero Theorem 2018 720p
- Reversing sail pdf
- Kzn Grade 12 September
- Dvd garth brooks
- Lying is the most fun a girl can have without taking her clothes off
- Punk rock compilation
- Natsuyuki rendezvous horriblesubs
- Predator 720 1987
- Situation amoureuse cest compliqu
- In live concert at the royal
- Inthevip dani daniels
- Lost s01e01 720p
- Company of heroes opposing
- Secrets of street magic
- Comedy central presents dave
- Relacions en el entorn del treball grau superior
- Tainted Blood Generation V 3
- Le nouvel edito b2 didier pdf
- Blood Ritual samael
- Paranormal witness s01e04
- Your First Move Chess for Beginners
- Soup 07 16
- La trama espaol
- Tesco Swot Analysis 2015 Pdf
- Free 480 12
- Iggy azalea new classic
- Termostato Perry Manuale Istruzioni Pdf
- At Midnight 2018 10 15
- Digital tutors digital painting
- Chavez
- Adventure time art
- Frozen 2018 cro
- Download game gta san andreas 5 for pc
- Filth the movie
- Leone sergio french
- Grace jones nightclubbing deluxe
- Injustice gods among us
- The fish that saved pittsburgh
- Naruto latest episode
- George of the jungle
- Bal sagoth flac
- Bruno mars lighters
- Bitdefender antivirus plus 2017 build
- Jefferson airplane vinyl
- Of sons of anarchy
- Sherlock holmes cast
- Humansoftware hsc edit
- Lets Be Cops xvid
- The jam disco
- Disneys the wild
- Russian institute lesson 4
- Cee lo f
- Mortal kombat armageddon pal
- The Kentucky Cycle
- Prayer secrets in the tabernacle
- Big brother after dark s16e02
- Set up 2
- Dont tell me its over
- The mermaid garden
- Remember me 2018
- Sleep hollow s02e04
- Trainz simulator
- Chamillionaire ammunition ep
- The road vostfr
- Adult Guidance 3
- Ministry of sound pump it up
- Perhubungan pekerjaan pdf
- Vmware cbt nugget
- The Greatest Hits N Spits